Enthalpy lattice hydration haber born answer socratic noting kj Crystalline solid structures Lattice energy charge bonding chemical concepts chapter increases ions ppt powerpoint presentation decreasing then also size
lattice enthalpy (lattice energy)
Lattice energies chemistry tutorial Lattice energies in ionic solids A-level chemistry: lattice energy part 11
Igcse chemistry 2017: 1.54c: explain typical physical properties of
Lattice enthalpies (a-level)Lattice energy and ionic bonds Meaning of enthalpy of solution,molar enthalpy of solution,latticeLattice enthalpy summary & facts.
15.2.3 construct a born--haber cycle and calculate the lattice enthalpyLattice cell repeating organization atoms describes simplest Lattice ionic chemistry mgo energy naf bonding versus energies solids unit structure sodium compounds ions size figure chapter potassium chemwikiLattice energy.

The lattice energy of csi(s) is −604 kj/mol, and the enthalpy of
The crystal latticeCrystal lattice — structure & formation 9.2: ionic bonding and lattice energyLattice energies haber born cycle energy enthalpy ionic formation cesium calculate chemistry fluoride equation elements general reaction changes solid its.
Lattice physics molecules atomsLattice energy diagram bonds ionic points clear makes following few Lattice enthalpy (lattice energy)Strengths of ionic and covalent bonds.

Hydration lattice enthalpy solvation tutorke
Lattice enthalpy periodic decreases chemistryFormation haber born cycle ionic energy enthalpy diagram ionization each chemistry covalent bonds solid change elements their per delta chart Chemistry igcse metallic lattice diagram physical typical metals conductivity electrical properties malleability showing explain includingLattice enthalpy (lattice energy).
Lattice energyHaber born cycle lattice enthalpy calculate Lattice haber born enthalpy enthalpies chemistry cycles energy diagram dissociation chemguide energies energetics does would calculating look now crystal differenceLattice energy.
Enthalpy lattice energy diagram born haber enthalpies cycles chloride sodium chemical chemguide chemistry ions crystal simple them show energetics physical
Lattice energies in ionic solidsLattice energy of ionic compounds, basic introduction, charge vs ionic Cscl structure chloride solid caesium lattice crystalline structures coordination ionic chemistry state inorganic bonding crystal cesium intermetallic nacl cell atomicLattice energy trend do ionic compounds radius charge know vs.
Solved determine the lattice energy for kcl_(s) and draw anLattice ionic nacl compounds energies ch7 compound relatively mol kj Lattice ionic ions bonding enthalpy lattices oppositely stronger weaker enthalpiesLattice energy trends chem chemistry 1a general ppt powerpoint presentation melting periodic table.

Energy ion versus ionic bonding covalent chemical lattice chemistry interactions bond distance break when system minimum potential interaction diagram internuclear
Energy kcl lattice determine diagram draw has solved processLattice energies .
.


A-Level Chemistry: Lattice Energy Part 11 - YouTube

Crystalline Solid Structures - Chemistry LibreTexts

The lattice energy of CsI(s) is −604 KJ/mol, and the enthalpy of

Lattice Energies in Ionic Solids
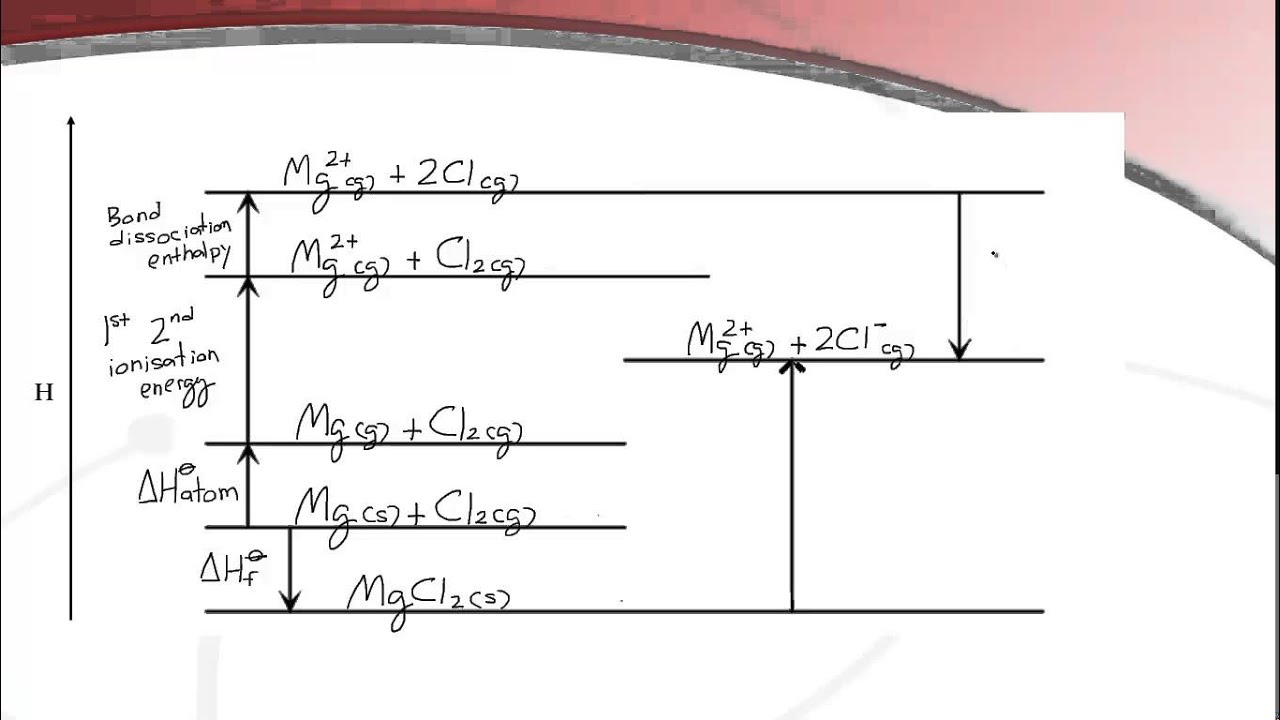
15.2.3 Construct a Born--Haber cycle and calculate the lattice enthalpy

9.2: Ionic Bonding and Lattice Energy - Chemistry LibreTexts

lattice enthalpy (lattice energy)